Integrated Lab Dashboard
Comprehensive analytics for Integrated Lab Testing
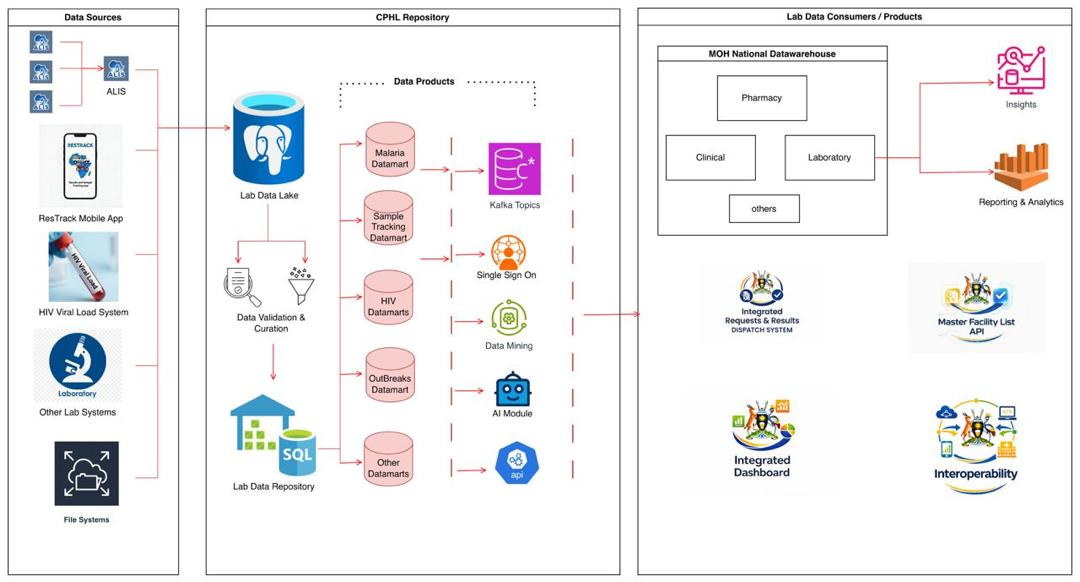
Lab Data Repository (LDR)
A Laboratory Data Repository (LDR) is a centralized system that collects, stores, and manages laboratory data from multiple laboratories and Laboratory Information Systems (LIS/LIMS) in one secure location. It enables the aggregation and standardization of test results, sample information, and related metadata, making laboratory data easily accessible for analysis, reporting, and decision-making. By consolidating historical and real-time laboratory data, an LDR supports disease surveillance, outbreak detection, quality assurance, research, and national health reporting. It reduces data silos, improves data quality and consistency, and provides a reliable foundation for dashboards, analytics, and public health intelligence across health systems.
Integrated with Ministry of Health National Data Warehouse
Actively Pushing Laboratory Data
Multi-Source Laboratory Data Ingestion
Collecting data from multiple lab programs
Data Integration
Unified view of ALIS facilities across Uganda's healthcare network
LDR Architecture
System architecture and data flow diagram
Exploration Framework
Surveillance
ALIS
ALIS system integration and data management.
Data Integrity97.4%
HIV
Early Infant Diagnosis
Early detection of HIV in infants for timely intervention.
Data Integrity90.1%
Surveillance
Genomics
Genomic sequencing and molecular epidemiology.
Data Integrity93.0%
HIV
Hepatitis B & C
Viral hepatitis testing and monitoring for co-infection management.
Data Integrity92.7%
HIV
HIV DR
HIV Drug Resistance monitoring and analysis.
Data Integrity90.7%
HIV
HIV Viral Load
Monitor viral suppression and treatment efficacy across all facilities.
Data Integrity93.2%
Surveillance
IMS (Inventory Management System)
Inventory management and supply chain tracking.
Data Integrity93.0%
Outbreak
Microbiology
Microbiological testing and pathogen identification.
Data Integrity93.0%
Outbreak
Outbreaks
Disease outbreak surveillance, tracking, and response coordination.
Data Integrity94.5%
Surveillance
Sample Tracking
Sample tracking and logistics management.
Data Integrity96.7%
NCD
Sickle Cell Disease
Newborn screening and hydroxyurea therapy monitoring.
Data Integrity96.8%
Outbreak
Tuberculosis
TB diagnosis, drug susceptibility testing, and treatment monitoring.
Data Integrity90.9%
